The Certified Nurse Medical Affairs Professional (CNMAP™) Program

Accredited by IACET/ANSI
The Certified Nurse Medical Affairs Program (CNMAP™) is a self-paced, online, certification program that provides nurses and advanced degree nurses the opportunity to educate themselves in the areas of medical affairs and life sciences, and therefore, creating a new avenue to career development. The CNMAP™ program enhances understanding of the medical affairs landscape within the pharmaceutical industry, allowing nursing professionals to power and advance their career in this rapidly expanding field.

15 Modules
6 Months Access
100% Online & Self Paced
Earn CME/CE Credits
Learning Objectives
Diversity in Clinical Trials
Gain an understanding of the current state of diversity in clinical trials and identify potential solutions to address underrepresentation in certain population segments.
Exploring the Biopharmaceutical Industry
Explore the structure, operations, and external factors governing the biopharmaceutical industry.
Medical Device & Diagnostics Industries
Develop insights into the medical device and diagnostics industries, including product influencers, trends, regulatory controls, and industry dynamics.
The Drug Development Process
Acquire comprehensive knowledge about the drug development process, from initial discovery to the marketplace, and understand the requirements for each phase in the development cycle.
Designing Effective Clinical Trials
Deepen understanding of how companies design and execute scientifically valid clinical trials, including various trial design types such as parallel studies, placebo-controlled trials, and observational trials.
Pharmaceutical Compliance
Familiarize yourself with pharmaceutical industry compliance rules and regulations, and understand the functions and responsibilities within pharmaceutical, biotech, medical device, or diagnostics companies.
Regulatory Affairs in Life Sciences
Gain an overview of the regulatory affairs function within life sciences organizations, both domestically and internationally, and understand its significance in ensuring compliance with regulations.
Master These Skills

All ACMA certifications are accredited by IACET/ANSI
25k+
Users
80+
Countries
250+
Companies
Module Breakdown
Module 1
Introduction to the Certified Nurse Medical Affairs Professional (CNMAP) Program
Clinical trials continue to underrepresent certain segments of the population. The objective of this course is to provide a better understanding of the current state of diversity in clinical trials, its causes and potential solutions. It also aims to present some solutions to common barriers preventing more diverse clinical trial participation.
Module 2
Biopharmaceutical Industry
Module 2 explores the pharmaceutical industry’s structure and operations, the divisions within pharmaceutical companies, and the external factors governing the industry.
Module 3
Device & Diagnostics Industries
Module 3 provides an overview of the medical device and the diagnostics industries, product influencers, trends, and regulatory controls. It also outlines the dynamics of these industries and how they differ from other life sciences industries.
Module 4
Drug Development Process
Module 4 presents an in-depth explanation of how a compound evolves from the initial discovery stage to the marketplace, detailing the requirements for each phase in the development cycle.
Module 5
Clinical Trial Design
Module 5 provides an in-depth understanding of how companies design clinical trials to generate scientifically valid results. It also reviews various clinical trial design types such as parallel studies, double blinded placebo controlled trials, cross sectional studies, meta analyses, systematic reviews, and observational trials.
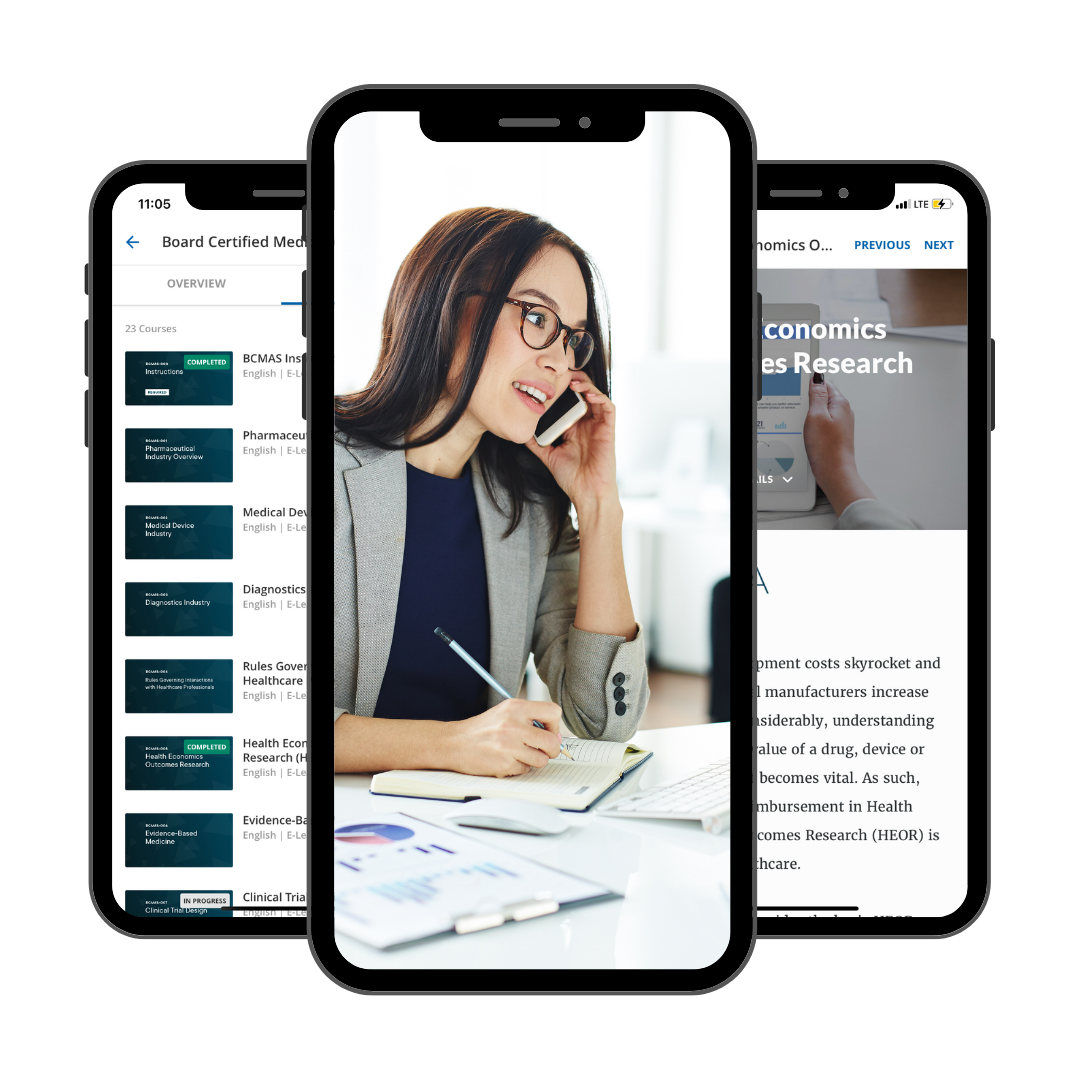
Download Our Learning Platform App
Go.Learn lets you to access our LMS platform directly from your mobile devices. Through the app, you can easily check programs, complete training material, and view various assets and channels.